Abstract
Introduction
Response criteria for judging the success of PV therapeutic interventions put forth by the ELN (Barosi Blood 2013 ) require normalization of blood counts and splenomegaly for 3 months following the administration of an agent. These criteria are frequently employed as a primary outcome in clinical trials, yet, the clinical significance of meeting these criteria have not been established. The goal of this study is to evaluate the prognostic impact of ELN response criteria in a large US retrospective database of PV patients (Ronner Blood 2020).
Methods
Included patients were age ≥18 years at diagnosis, met 2016 WHO diagnostic criteria for PV, and were treated with a cytoreductive agent including hydroxyurea (HU), pegylated-interferon or ruxolitinib for at least 12 weeks. Laboratory and spleen measurements were collected throughout the treatment course. Attaining an ELN response throughout the study period was defined as concurrent WBC <10 x 10^9/L, hematocrit <45%, platelet <400 x 10^9/L, and lack of palpable splenomegaly for a minimum of 12 consecutive weeks.
Multivariable Cox proportional hazards methods were used to determine the associations between achieving an ELN response and events such as thrombosis, disease progression (development of myelofibrosis [MF], myelodysplastic syndrome [MDS], or myeloproliferative neoplasm in blast phase [MPN-BP] as clinically determined by treating physician) and death. Time-dependent Cox proportional hazards models were also assessed to examine the relationship between duration of response and these outcomes. All models were adjusted for age, gender, history of prior thrombosis, and the presence of cardiovascular (CV) risk factors.
Results
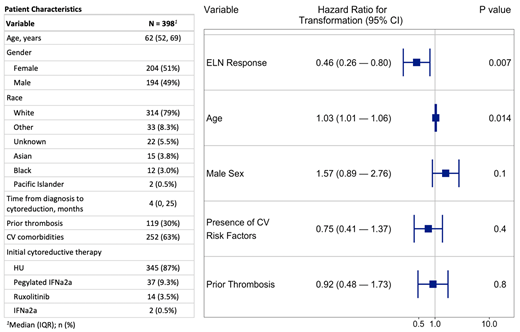
A total of 398 of the 527 patients received cytoreductive therapy for at least 12 weeks and were included in the current analysis. Baseline characteristics are shown in the Table. HU was the most common initial cytoreductive agent employed. The median maximum dose of HU was 1000mg daily (IQR 714-1500).
During a median follow-up of 52.3 months, 249 (63%) patients attained an ELN response and 149 (37%) did not. The median duration of response was 37 weeks (IQR 16-74). Forty-five patients (11%) experienced a new thrombosis, including DVT (n=15), CVA/TIA (n=14), PE (n=5), MI (n=6), and splanchnic vein thrombosis (n=6). Disease progression occurred in 51 (13%) patients, including MF (n=47), MDS (n=3), and MPN-BP (n=1). Thirteen patients (3%) died during follow up.
In our Cox-proportional hazards model, meeting an ELN response was not associated with a reduced risk of developing a thrombosis (p=0.86), but having a prior thrombosis was associated with acquiring a subsequent thrombosis (HR 3.3, 95% CI 1.80-6.05). Similarly, ELN responses were not associated with reduction in hazard of death (p=0.80). However, an ELN response was associated with a significant decrease in the hazard of progression (HR 0.47, 95% CI 0.27-0.83) (Figure). Advanced age at diagnosis was also associated with an increased hazard of progression (HR 1.03, 95% CI 1.01-1.05). Similar results were observed when examining the relationship of ELN response duration as a time-dependent covariate as it relates to thrombosis (HR 0.82, 95% CI 0.41-1.65), progression (HR 0.39, 95% CI 0.17-0.90) and death (HR 0.82, 95% CI 0.23-2.88).
We also evaluated the contribution of individual ELN response components using a multivariable Cox model adjusted for age, sex, prior thrombosis, and CV risk factors. The decrease in hazard of progression was largely driven by two components: WBC <10 x 10^9/L (HR 0.28, 95% CI 0.14-0.56) and absence of splenomegaly (HR 0.25, 95% CI 0.12-0.52). Interestingly, achieving a platelet count of <400 x 10^9/L was associated with a significant increase in the hazard of progression (HR 2.70, 95% CI 1.26-5.80) while achieving a hematocrit response was not associated with progression (HR 1.91, 95% CI 0.80-4.55).
Conclusions
Achieving an ELN response in PV patients is not associated with a reduction of risk for thrombosis or death but may be a surrogate endpoint for delaying disease progression. When evaluating new therapeutic strategies with the primary goal of reducing thrombotic burden in PV, the present ELN response criteria are not informative. The development of new reliable surrogate endpoints for predicting thrombotic risk are required in order to rapidly evaluate interventions that can prevent thrombosis in PV patients.
Podoltsev: Incyte: Honoraria; Novartis: Honoraria; Blueprint Medicines: Honoraria; Pfizer: Honoraria; PharmaEssentia: Honoraria; CTI BioPharma: Honoraria; Bristol-Myers Squib: Honoraria; Celgene: Honoraria. Gotlib: Allakos: Consultancy; Cogent Biosciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Chair for the Eligibility and Central Response Review Committee, Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Heaney: Cogent: Research Funding; CTI: Honoraria, Research Funding; BMS: Research Funding; Blueprint: Honoraria, Research Funding; Sierra Oncology: Research Funding; Kartos: Research Funding; Novartis: Honoraria. Kuykendall: Abbvie: Honoraria; Blueprint: Honoraria; Novartis: Honoraria, Speakers Bureau; Incyte: Consultancy; Protagonist: Consultancy, Research Funding; Pharmaessentia: Honoraria; Celgene/BMS: Honoraria. Shammo: Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; CTI pharma: Research Funding; Stemline therapeutics: Research Funding; Kartos Pharma: Research Funding; Takeda: Consultancy, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Honoraria, Research Funding; Abbvie: Current holder of individual stocks in a privately-held company, Research Funding; Baxter: Current holder of stock options in a privately-held company; sanofi: Consultancy, Honoraria, Speakers Bureau; NS Pharma: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Astra zeneca: Research Funding. Mesa: La Jolla Pharma: Consultancy; Incyte Corporation: Consultancy, Research Funding; CTI: Research Funding; Samus: Research Funding; Pharma: Consultancy; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy; AOP: Consultancy; Promedior: Research Funding; Constellation Pharmaceuticals: Consultancy, Research Funding; Abbvie: Research Funding; CTI: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Genentech: Research Funding. Yacoub: Incyte: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; ACCELERON PHARMA: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dynavex: Current equity holder in publicly-traded company; Cara: Current equity holder in publicly-traded company; Ardelyx: Current equity holder in publicly-traded company; Seattle Genetics: Honoraria, Speakers Bureau; Hylapharm: Current equity holder in publicly-traded company. Hoffman: Protagonist Therapeutics, Inc.: Consultancy; Kartos Therapeutics, Inc.: Research Funding; Novartis: Other: Data Safety Monitoring Board, Research Funding; AbbVie Inc.: Other: Data Safety Monitoring Board, Research Funding. Mascarenhas: AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Research Funding; Merus: Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Prelude: Consultancy; Promedior: Consultancy, Membership on an entity's Board of Directors or advisory committees; Galecto: Consultancy; CTI Biopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forbius: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract